Disinfectant / Antimicrobial Efficacy Studies

BACKGROUND:
In Australia the testing regimens set out in Therapeutic Goods Order no. 104 (TGO 104) Order 2019 may be used to demonstrate compliance with the essential principles related to performance of a disinfectant. The use of Therapeutic Goods Order no. 104 (TGO 104) Order 2019 is not mandatory and a manufacturer may choose to use other standards, or tests for performance. However, the manufacturer must be able to demonstrate that other tests used deliver an outcome at least equivalent to the testing specified in Therapeutic Goods Order no. 104 (TGO 104) Order 2019. Antiseptics are treated as medicines in Australia there are not such clear guidelines as there are for disinfectants. A combination of in vitro efficacy and hand wash/rub studies are usually sufficient for registration purposes.
PRINCIPLE OF TEST:
Disinfectant is prepared according to the manufacturer’s recommendations. They are then tested by either a suspension or carrier format procedure. For the suspension format, the product at recommended dilution is inoculated with a known level of specified organisms. After a specified contact time, the suspension mixture is then neutralized with a validated neutralizer and any surviving organisms is recovered. For the carrier format, an inert carrier is inoculated with organisms which are dried and then the carrier is added to the product. Again, after the specified contact time the carriers are assessed for growth or no growth.
There are several test methods available depending on the manufacturer’s proposed claim for a disinfectant.
Time Kill Study / Antimicrobial Activity Test:
The study of the time required for a certain product concentration to kill certain level of bacteria/ fungi. Eurofins BPT Sydney test protocol code is TMD-110
TGA Test (Option A, Option B, Option C and Option D):
Option A and B are for products with a label claim of Hospital grade; Option C is for products with a label claim of Household/Commercial grade and Option D is for products with a label claim of Antiseptic. Note that antiseptics are not covered by Therapeutic Goods Order no. 104 (TGO 104) Order 2019 requirements. Eurofins BPT Sydney test protocol code is TMD-122
Hard Surface Carrier:
This test is to demonstrate the effectiveness of a disinfectant on a certain contaminated surfaces. Eurofins BPT Sydney test protocol code is TMD-253, based upon the AOAC method.
Germicidal Spray Test:
This test is to determine effectiveness of sprays and pressurized spray products as spot disinfectants for contaminated surfaces. Eurofins BPT Sydney test protocol code is TMD-254, based upon the AOAC method.
Sporicidal Carrier and Suspension Test:
This test is to determine the sporicidal activity of a product against specified spore-forming bacteria in various situations and potential efficacy as sterilizing agent. Eurofins BPT Sydney test protocol code is TMD-257 and TMD-250 respectively, based upon AOAC and ASTM methods.
Quantitative Mycobacteria Carrier:
This assesses the efficacy of disinfectants and sterilants against Mycobacterium terrae by using the quantitative carrier method. Quantitative suspension test formats similar to the Ascenzi method are also available if required. Eurofins BPT Sydney test protocol code is TMD-250.
Fungicidal Test:
This determines the sample dilution required to kill a certain level of Trichophyton mentagrophytes in ten minutes. This test is an adaption of the phenol co-efficient test. Eurofins BPT Sydney test protocol code is TMD-255, based upon the AOAC method
Simulated In-Use Test:
Tests under this guideline are customized to test the effectiveness of the product in ' real-life application ' and conditions under which the product would be used. Typically, the test procedure would follow the ' Directions for Use' as described on the product label, or designed in consultation with client to mimic the normal operating process.
Hand rub and Hand wash Studies:
These are volunteer studies, where simulated in-use hand rub/wash procedures are used to evaluate the products Protocols used EN 1499 and EN 1500, EN12791.
Virucidal Studies:
We have a variety of reference viruses available for disinfectant evaluation of proposed marketing claims, includingAdeno virus, Herpes virus and Polio virus. Testing may be performed as a suspension or carrier format depending on the nature of the proposed claims. Methods are based upon ASTM standards.
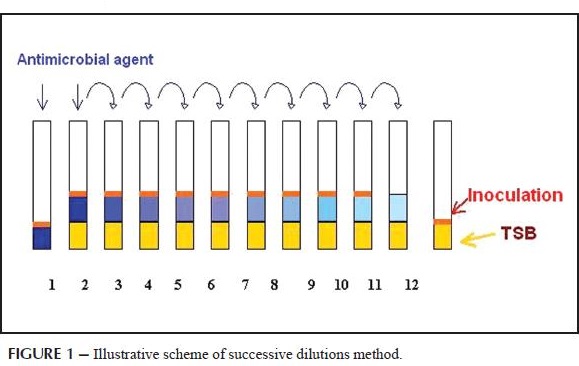
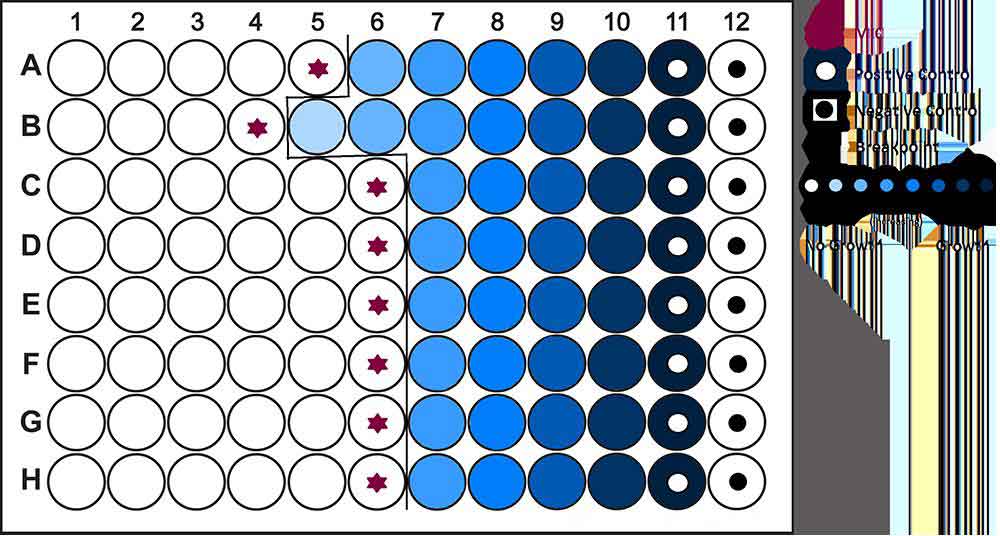
Minimum Inhibitory Concentration (MIC):
Minimum Inhibitory Concentration (MIC) is the lowest concentration of an antimicrobial (Antifungal, Antibiotic or Bacteriostatic) drug that will inhibit the visible growth of a microorganism after overnight incubation. MICs can be determined on plates of solid growth medium or broth dilution methods after a pure culture is isolated. For example, to identify the MIC via broth dilution, identical doses of bacteria are cultured in wells of liquid media containing progressively lower concentrations of the drug. The minimum inhibitory concentration of the antibiotic is between the concentrations of the last well in which no bacteria grew and the next lower dose, which allowed bacterial growth. There are also several commercial methods available to experimentally measure MIC values. An MIC is generally regarded as the most basic laboratory measurement of the activity of an antimicrobial agent against an organism. Because a lower MIC value indicates that less of the drug is required in order to inhibit growth of the organism, drugs with lower MIC scores are more effective antimicrobial agents. Clinicians use MIC scores to choose which antibiotics to administer to patients with specific infections and to identify an effective dose of antibiotic. This is important because populations of bacteria exposed to an insufficient concentration of a particular drug or to a broad-spectrum antibiotic (one designed to inhibit many strains of bacteria) can evolve resistance to these drugs. Therefore, MIC scores aid in improving outcomes for patients and preventing evolution of drug-resistant microbial strains.

Zone of Inhibition (ZOI):
Zone of inhibition is performed by both agar well or disc diffusion method against antibiotics and organisms of choice.
Any Other Studies:
We are happy to perform other studies according to client testing protocols under GLP conditions if requested. Please ask the laboratory for assistance/advice.
METHOD REFERENCE:
- AOAC Method 961.02 : 2005 - Germicidal Spray Products as Disinfectants.
- TGO No 54, 54A & 54B 2009 & Guidelines; Standard for Composition, Packaging, Labelling and Performance of Disinfectants, and Sterilants, 6.4.7.
- US EPA, Public Health Use Antimicrobial Agent Product Performance Test Guidelines, Sub-series 91A.
- AOAC Official Method 955.17, Fungicidal Activity of Disinfectants (2005).
- EN 13704:2002 Chemical Disinfectants Quantitative suspension test for the evaluation of sporicidal activity of chemical disinfectant.
- AOAC Method 966.04. Sporicidal Activity of disinfectants, 2007.
- ASTM: E 2315-03, Standard guide for Assessment of Antimicrobial Activity Using a Time Kill Procedure.
- EN1276, Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic, and institutional areas.
SAMPLE REQUIREMENTS:
Between 100ml to 2000ml in test dilution, depending on test protocols.
TURNAROUND TIME (TAT) – general guide , varies depending on method selection:
- Bactericidal, fungicidal and yeasticidal efficacy 14, 21 or 28 Working Days (WD)
- Virucidal Efficacy 40 Working Days (WD)
- Mycobacteria efficacy 65 Working Days (WD)
- Tuberculocidal efficacy 90 Working Days (WD)
- Automated Airborne Disinfection 25 Working Days (WD) from the Friday of the booked week for the Project.
to EN17272 in purpose-built Project Room

